Welcome to our comprehensive guide to naming covalent compounds worksheet answers. This guide is designed to provide you with a thorough understanding of the rules and conventions for naming covalent compounds, empowering you to confidently tackle any naming challenge.
We will delve into the various types of covalent compounds, including diatomic, triatomic, and polyatomic molecules. We will explore the systematic approach to naming binary covalent compounds, considering prefixes for indicating the number of atoms of each element. Additionally, we will unravel the concept of polyatomic ions, their naming conventions, and their role in naming polyatomic covalent compounds.
Types of Covalent Compounds: Naming Covalent Compounds Worksheet Answers
Covalent compounds are formed when atoms share electrons to achieve a stable electron configuration. Based on the number of atoms involved, covalent compounds can be classified as:
- Diatomic: Compounds consisting of two atoms, e.g., H2, Cl2
- Triatomic: Compounds consisting of three atoms, e.g., H2O, CO2
- Polyatomic: Compounds consisting of more than three atoms, e.g., NH3, CH4
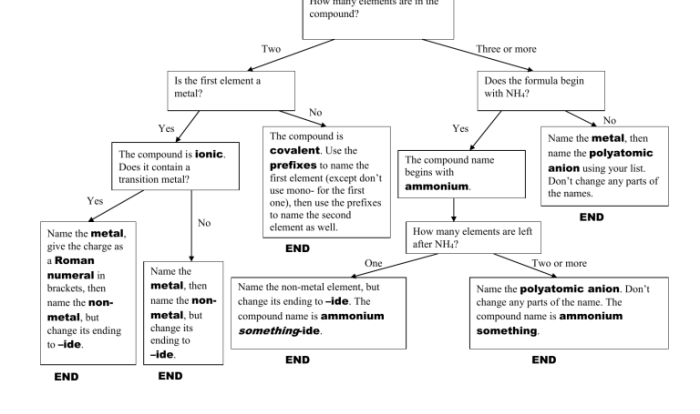
Naming Binary Covalent Compounds
Binary covalent compounds consist of two nonmetal elements. The naming follows these rules:
- Use the root name of the first element.
- Use the suffix “-ide” for the second element.
- Use prefixes to indicate the number of atoms of each element (mono-, di-, tri-, tetra-, etc.).
Examples:
- CO: carbon monoxide
- N2O: dinitrogen monoxide
- PCl5: phosphorus pentachloride
Naming Polyatomic Covalent Compounds
Polyatomic covalent compounds contain a polyatomic ion, which is a group of atoms that carries a charge. Common polyatomic ions include:
| Name | Formula | Charge |
|---|---|---|
| Ammonium | NH4+ | +1 |
| Hydroxide | OH- | -1 |
| Nitrate | NO3- | -1 |
To name polyatomic covalent compounds:
- Use the name of the cation (positive ion) first.
- Use the name of the polyatomic ion (anion) second.
Examples:
- NH4Cl: ammonium chloride
- NaOH: sodium hydroxide
- Ca(NO3)2: calcium nitrate
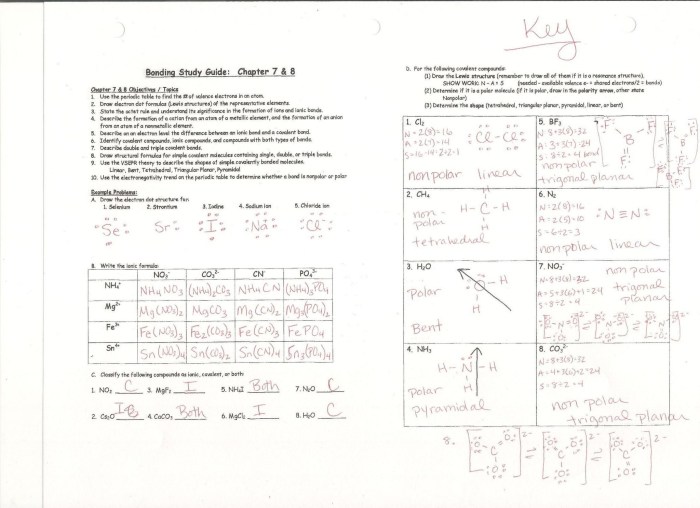
Exercises and Practice Problems
| Problem | Answer |
|---|---|
| Name the following compound: CO2 | Carbon dioxide |
| Name the following compound: NH3 | Ammonia |
| Write the formula for the compound named sulfur trioxide | SO3 |
Advanced Topics
Advanced topics in covalent compounds include:
- Resonance: The concept that certain covalent compounds can exist in multiple equivalent Lewis structures.
- Lewis structures: Diagrams that show the arrangement of atoms and electrons in a covalent compound.
- Molecular geometry: The three-dimensional shape of a covalent compound.
- Polarity: The uneven distribution of electrons in a covalent compound.
User Queries
What are the different types of covalent compounds?
Covalent compounds can be classified into diatomic, triatomic, and polyatomic molecules based on the number of atoms involved.
How do you name binary covalent compounds?
Binary covalent compounds are named using prefixes to indicate the number of atoms of each element, followed by the root name of the second element and the suffix “-ide”.
What are polyatomic ions?
Polyatomic ions are groups of atoms that carry a net charge and behave as a single unit. They have specific names and formulas.