Embark on a captivating journey through the history of atomic theory worksheet, where the evolution of our understanding of the fundamental building blocks of matter unfolds. From the ancient postulates of Democritus to the groundbreaking experiments of modern physicists, this comprehensive guide delves into the key concepts, models, and applications that have shaped our knowledge of the atom.
As we trace the historical development of atomic theory, we will explore the contributions of pioneering scientists who laid the foundation for our current understanding of the atom. We will examine the experiments that led to the discovery of subatomic particles and delve into the evolution of atomic models, from the Bohr model to the quantum mechanical model.
Along the way, we will uncover the practical applications of atomic theory in fields such as chemistry, physics, and medicine, while also considering the ethical implications of its use in nuclear energy and weapons.
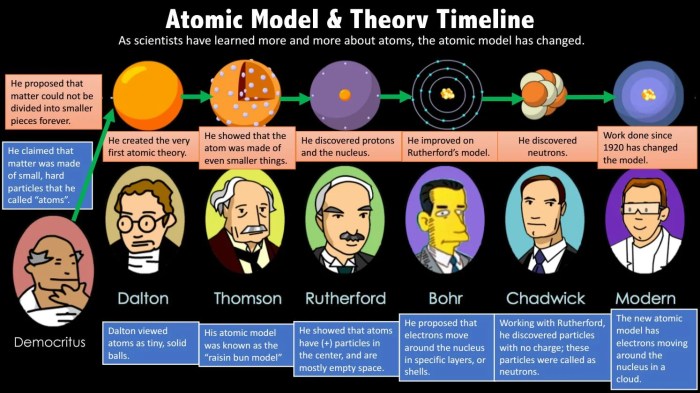
Historical Development of Atomic Theory
Atomic theory has evolved significantly over time, with key contributions from various scientists.
Contributions of Democritus and Leucippus
Democritus and Leucippus proposed the idea of atoms as indivisible, indestructible particles around 400 B.C. They believed that atoms were in constant motion and that their interactions gave rise to all matter.
John Dalton’s Modern Atomic Theory
In the early 19th century, John Dalton established the modern atomic theory, which proposed that:
- Matter is composed of tiny, indivisible particles called atoms.
- Atoms of the same element are identical in mass and other properties.
- Atoms of different elements have different masses and properties.
- Chemical reactions involve the rearrangement of atoms.
Discovery of Subatomic Particles
Later experiments by J.J. Thomson, Ernest Rutherford, and James Chadwick led to the discovery of subatomic particles:
- Thomson discovered the electron in 1897, demonstrating that atoms were not indivisible.
- Rutherford’s gold foil experiment in 1911 revealed the presence of a dense, positively charged nucleus within the atom.
- Chadwick discovered the neutron in 1932, completing the picture of the atom as a nucleus surrounded by electrons.
Key Concepts of Atomic Theory
An atom is the basic unit of matter, consisting of:
Protons
Positively charged particles in the nucleus.
Neutrons
Neutral particles in the nucleus.
Electrons
Negatively charged particles that orbit the nucleus.
Atomic Number and Mass Number
Atomic number
The number of protons in an atom, which determines its element.
Mass number
The total number of protons and neutrons in an atom.
Electron Arrangement
Electrons occupy specific energy levels around the nucleus, called orbitals. The arrangement of electrons in orbitals determines the atom’s chemical properties.
Evolution of Atomic Models
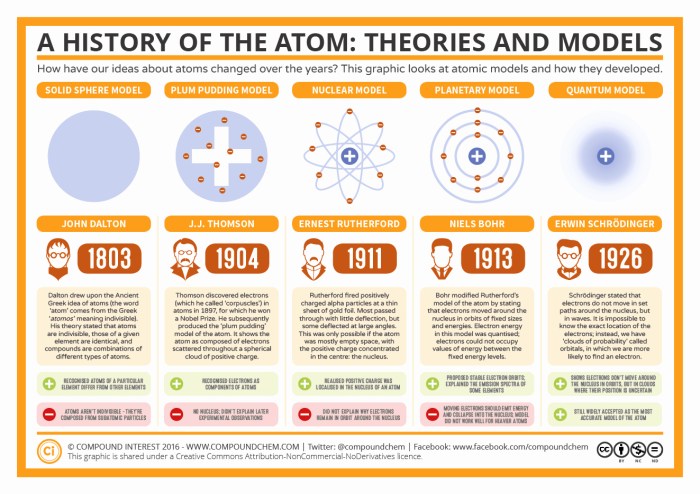
| Model | Strengths | Limitations |
|---|---|---|
| Bohr Model (1913) | – Introduced the idea of electron energy levels.
|
– Did not account for the wave-particle duality of electrons.
|
| Rutherford Model (1911) | – Introduced the concept of a dense, positively charged nucleus.
|
– Did not account for the stability of the atom.
|
| Quantum Mechanical Model (1926) | – Explained the wave-particle duality of electrons.
|
– Complex and difficult to solve.
|
Revolutionizing Our Understanding
The quantum mechanical model revolutionized our understanding of the atom by introducing the wave-particle duality of electrons and providing a more accurate description of atomic structure.
Applications of Atomic Theory
Atomic theory has wide-ranging applications in various fields:
Chemistry
- Understanding chemical bonding and reactions.
- Developing new materials with tailored properties.
Physics, History of atomic theory worksheet
- Explaining the properties of matter and energy.
- Developing new energy sources.
Medicine
- Understanding the structure and function of biological molecules.
- Developing new drugs and treatments.
Ethical Implications
Atomic theory has also raised ethical implications, particularly in the development of nuclear energy and weapons. It is crucial to consider the potential risks and benefits associated with these applications.
Question & Answer Hub: History Of Atomic Theory Worksheet
What is the basic unit of matter?
The atom is the basic unit of matter and the building block of all elements.
Who is credited with developing the first atomic theory?
Democritus and Leucippus are credited with developing the first atomic theory in ancient Greece.
What is the role of electrons in an atom?
Electrons are negatively charged particles that orbit the nucleus of an atom and determine its chemical properties.